Alpha Ferrite – Properties and Definition
Iron Allotropy: Allotropy is the property of a material to exist in different forms in the same, stable physical state. This property is widely attributed towards Diamond or graphite but Iron is also one of the finest examples of metals that show such a behavior. Iron is known to exist in three different forms at atmospheric pressure. These are Alpha Ferrite, Gamma Iron or Austenite and Delta Iron.
What separates these forms of Iron is the temperatures at which they are stable and the structure of the crystal lattice of Iron at these conditions. These factors play a major role in the solubility of Carbon in Iron and thus is vital to know when making different kinds of steel.
What is Alpha Ferrite: Alpha Ferrite is the phase which exists below temperatures of 912 degree Celcius for low concentrations of Carbon in Iron. Alpha Ferrite can only dissolve up to 0.02 percent of Carbon at 727 degree Celcius. This is because of the configuration of the iron lattice which forms a BCC crystal structure.
BCC stands for Body Centred Cubic structure in which there is an iron atom present in the center of a unit cell and at each corner of the cell. This configuration does not provide many interstitial spaces for Carbon atoms to fill so the solubility is low.
Properties of Alpha Ferrite: Alpha Ferrite is the stable form of Iron at room temperature. It is soft and ferromagnetic at temperatures below 727 degrees and paramagnetic between 727and 912 degrees. Alpha ferrite is a large component of mild steel but is present in all Iron carbon compounds that have been cooled to room temperature, co existing with other forms such as Cementite.
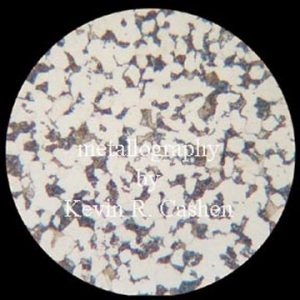
Credits: Kevin R. Cashen