What is Propylene – Technical & User Specifications
What is Propylene? Propylene is a member of the Alkene family of hydrocarbons. It is an unsaturated compound with the chemical formula of C3H6. It exists in its pure form as a colorless gas with a slight smell like petroleum. It is volatile in nature and easily flammable therefore it is used as a fuel in Oxyfuel welding. It is used as a liquefied gas but more importantly it is used as the building block of other compounds and polymers.
How is Propylene produced? Propylene is produced by the cracking of larger hydrocarbons from natural gas and oil. A large portion of this comes from the cracking of naphtha under the action of steam or the cracking of propane and butane. Propylene is produced mostly from petroleum and natural gas as well as a little amount from coal.
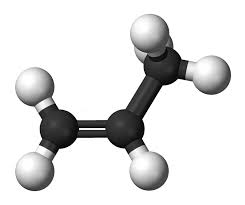
Structure and Applications of Propylene: Propylene is a simple hydrocarbon with 3 Carbon atoms and six Hydrogen atoms. Its structure contains one double bond between Carbon atoms which is responsible for giving it much of its reactivity which enables it to be used in the production of other compounds.
The majority of propene reactions are addition reactions in which atoms are added to the double bond. This is the process which is used to form the widely used polymer polypropylene. Additionally, Propylene can also undergo substitution reactions which involve its methyl group which has weaker bonds than the other C-H bonds.
These properties enable Propylene to be used in the production of Propenal which is in turn oxidized to form Propenoic acid that is used in the production of acrylic polymers. It is also used to make Cumene which is used to make phenol and acetone.
 Propylene or Propene is also used as fuel in some types of oxyfuel welding as a substitute for oxyacetylene. Propylene gas provides a combination of the properties of acetylene and propane. The burning temperature of Propylene is greater than that of Propane while is qualities are similar to acetylene. The BTU capacity of of the outer flame is also better than that of acetylene.
Propylene or Propene is also used as fuel in some types of oxyfuel welding as a substitute for oxyacetylene. Propylene gas provides a combination of the properties of acetylene and propane. The burning temperature of Propylene is greater than that of Propane while is qualities are similar to acetylene. The BTU capacity of of the outer flame is also better than that of acetylene.